SSC CGL - Detailed Guide 2025
Self-Paced Course

Chemical Bonding and Reactions
Reference: Lucent GK, NCERT Class 6–12
Types of Bonds: Ionic & Covalent
Ionic Bond
| Feature | Description |
|---|---|
| Definition | Electrostatic force of attraction between oppositely charged ions |
| Occurs Between | Metal (loses e⁻) and Non-metal (gains e⁻) |
| Electron Transfer | Complete transfer from metal to non-metal |
| Properties | High melting & boiling point, soluble in water, conduct electricity in solution |
| Example | NaCl (Na⁺ + Cl⁻), CaCl₂ |
Metal atoms lose electrons to form cations, non-metals gain to form anions.
Covalent Bond
| Feature | Description |
|---|---|
| Definition | Sharing of electrons between atoms |
| Occurs Between | Non-metal and non-metal |
| Electron Transfer | No transfer; shared pairs of electrons form bonds |
| Properties | Low melting & boiling point, poor conductor, often insoluble in water |
| Example | H₂, O₂, CO₂, CH₄ |
Can be single (H₂), double (O₂), or triple (N₂) covalent bonds.
Chemical Equations and Balancing
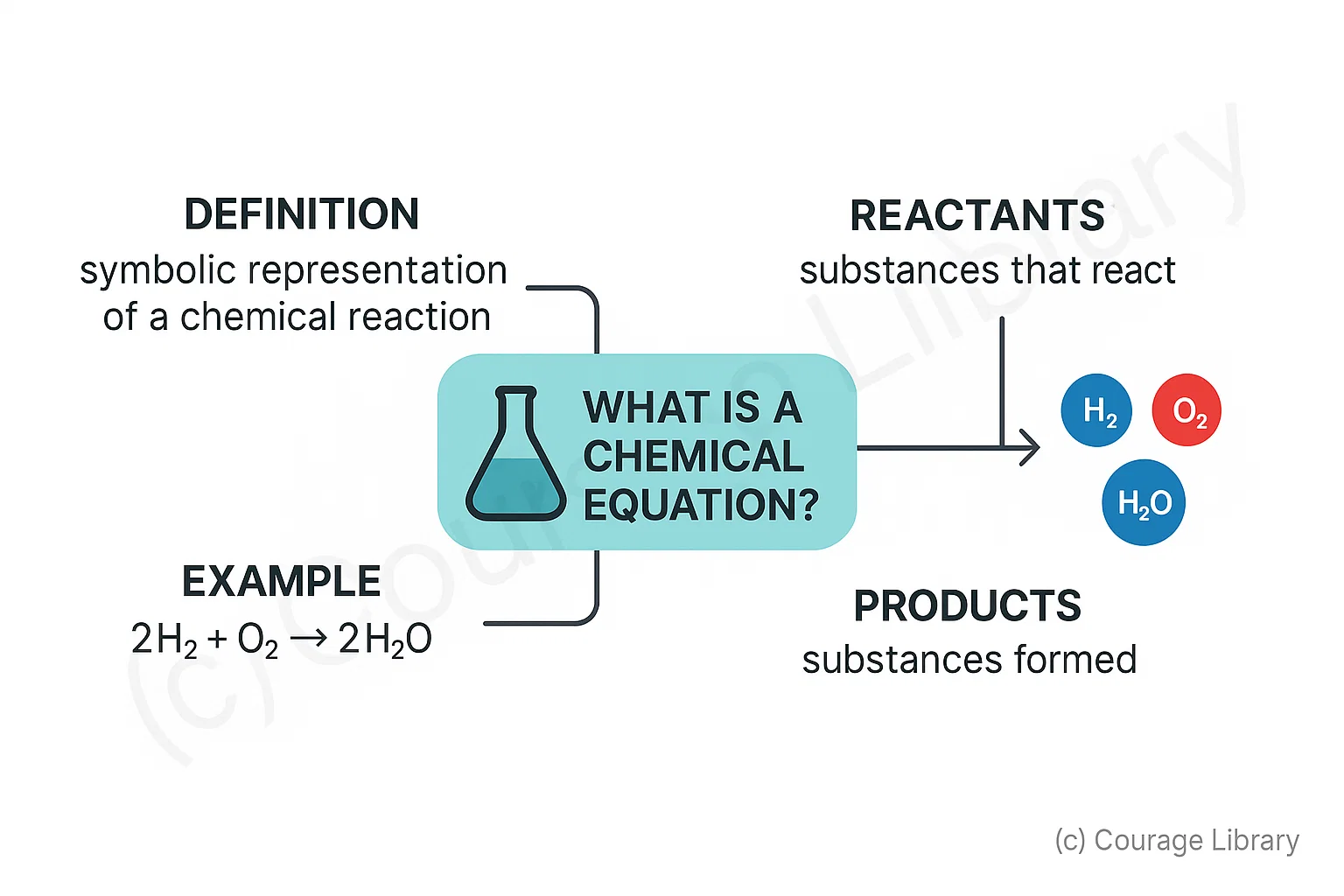
What is a Chemical Equation?
- A symbolic representation of a chemical reaction showing reactants & products
-
Example: 2H₂ + O₂ → 2H₂O
- Reactants: Substances that react
- Products: Substances formed
Balancing Chemical Equations
| Step | Description |
|---|---|
| 1. Write skeleton equation | Unbalanced equation with formulas |
| 2. Count atoms | Compare number of atoms on both sides |
| 3. Balance using coefficients | Adjust numbers (not subscripts) to equal atoms |
| 4. Double check | Recount to ensure balance is correct |
Follows Law of Conservation of Mass: mass of reactants = mass of products.
Types of Reactions
| Type | Description | General Form | Example |
|---|---|---|---|
| Combination | Two or more substances combine to form one product | A + B → AB | H₂ + Cl₂ → 2HCl |
| Decomposition | One compound breaks into two or more simpler substances | AB → A + B | 2H₂O₂ → 2H₂O + O₂ |
| Displacement | One element displaces another in a compound | A + BC → AC + B | Zn + CuSO₄ → ZnSO₄ + Cu |
| Double Displacement (exchange reaction) | Two compounds exchange ions to form two new compounds | AB + CD → AD + CB | NaCl + AgNO₃ → NaNO₃ + AgCl↓ |
Developed Roopasree Challa
Next
Start Your SSC CGL Journey Now!
Join Courage Library to experience disciplined study and expert support.
Be a Couragian!